Location : Bordeaux (University Hospital) Focus : Emergency and Post-Operative Recovery (Cardiac Surgery). Overview The clinical trial at Hospital Pellegrin targets one of the most critical windows in patient care: the immediate post-operative period following cardiac surgery (e.g., valve replacement, CABG). This study investigates the « Bridge to Home » concept, aiming to safely reduce hospital stays […]
Location : Bordeaux Lead Investigators : Dr. Eric Abergel & Dr. Stéphane Garrigue Study Protocol This pivotal validation study was designed to prove the core innovation of Medrik Technology: the ability to generate hemodynamic data non-invasively. Unlike standard fitness trackers that only measure heart rate, this study focused on complex volumetric metrics usually reserved for […]
Location : Pessac, Bordeaux Area (CHU de Bordeaux) Focus Area : Advanced Electrophysiology and Heart Failure Management. Study Protocol The clinical trial at Haut-Lévêque Hospital represents a critical phase in validating the Medrik Ring for long-term ambulatory monitoring. This facility, renowned for its cardiology and electrophysiology departments, provides the ideal environment to test the ring’s […]
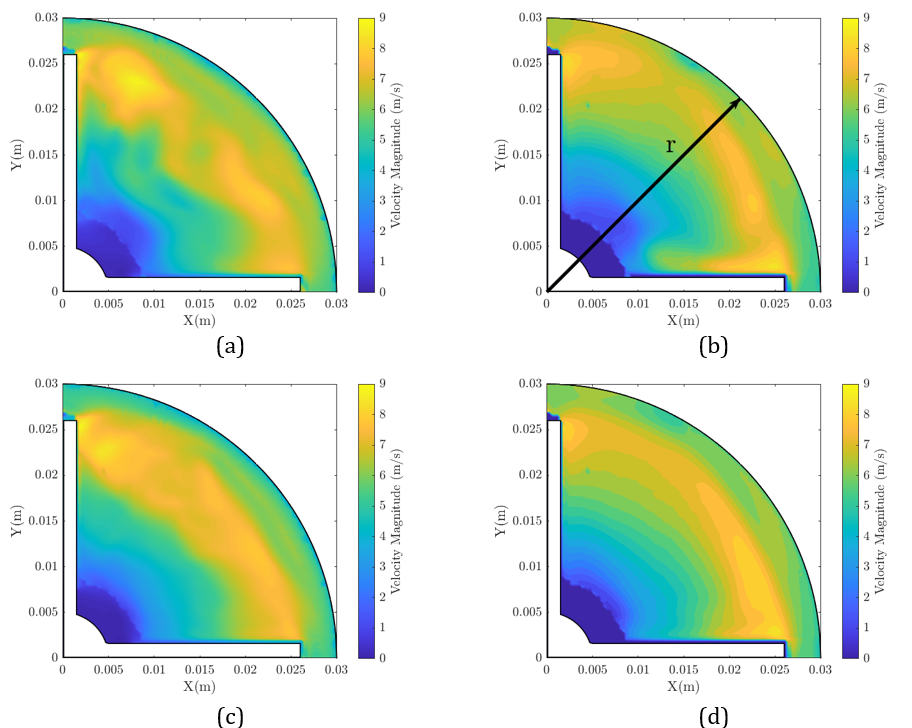
Developing blood-contacting medical devices like ventricular assist pumps demands rigorous testing. Not only must the device deliver the right flow and pressure, but it also needs to minimize red blood cell damage (hemolysis). Predicting hemolysis early in the design phase can save time and reduce costly prototype testing. One of the most reliable ways to […]
When it comes to developing blood-contacting medical devices such as ventricular assist devices (VADs), the ability to accurately predict flow and hemolysis inside rotating pumps is critical. After validating our computational approach on the simpler FDA Nozzle Benchmark, we moved on to the more challenging FDA Pump Benchmark — a full centrifugal blood pump model […]